Endometrial Microbiome Test
Endometrial Microbiome Test discover more about your pregnancy
What is Endometrial Microbiome ?
Just like skin microbiota, many bacterial species coexist in the reproductive organs such as the vagina and uterus, this is the Endometrial Microbiome. Good bacteria such as Lactobacillus are known to protect the fetus from viral infections and pathogenic bacterial infections by creating an environment where other bacteria cannot multiply. High levels of Lactobacillus have been linked to an increase in successful embryo implantation and live births.
While it was previously considered was the uterus was sterile, researchers at Rutgers University discovered in 2015 that Lactobacillus are present in the endometrium. Dr. Moreno of Stanford University reported in 2016 that dysbiosis in the endometrial microbiota can cause unfavorable IVF outcomes.
It suggested that the host immune system may be activated by pathogens to attack the fertilized egg as a foreign body.
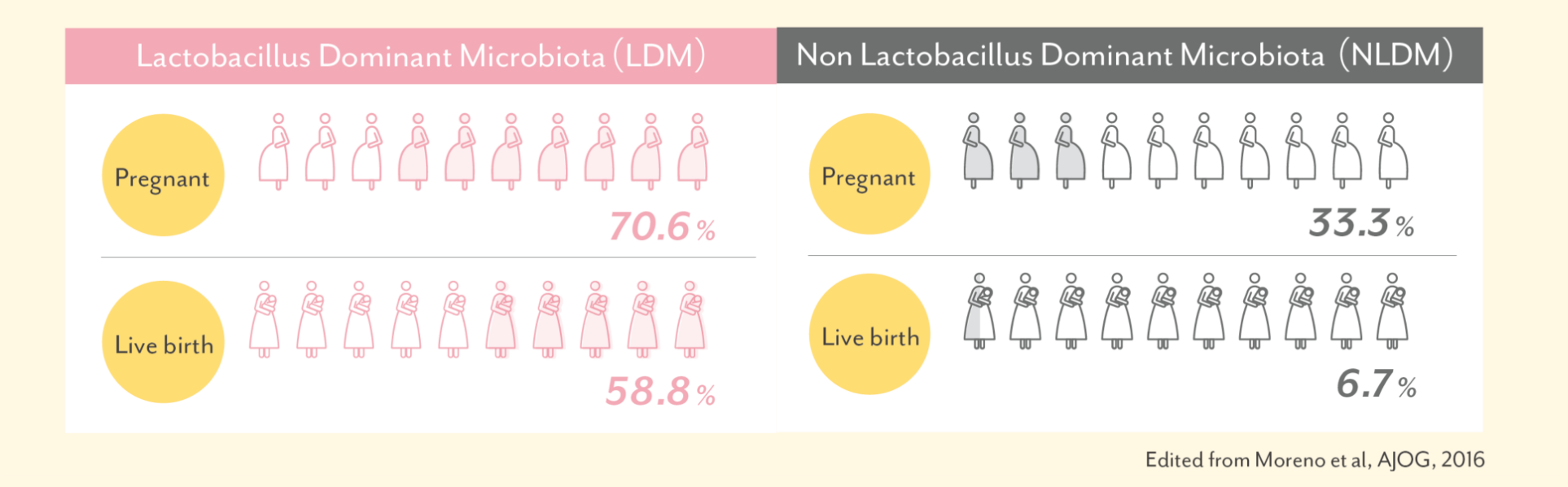
The environment in the uterus has an effect on pregnancy and livebirth rates
According to the investigation at IVI Valencia Clinic, women having more than 90% of endometrial Lactobacillus(LDM)
had higher pregnancy and live birth rates compared towomen having lower percentage of endometrial Lactobacillus(NLDM).
What is Endometrial Microbiome Test?
Varinos is the first company in the world to commercialize an Endometrial Microbiome test
The endometrial microbiome test examines the existence of Lactobacillus spp in the endometrium or vagina. There have been report s correlating the existence of Lactobacillus spp in the uterus with pregnancy outcomes.
The percentage contributions of other bacterial species are also provided including pathogenic bacterial species. Results are presented in an easy interpret report for assessment of the microbiome makeup of the sample.
Please note that this is not a diagnostic test.
Patient
-
Women planning to
conceive in the future
-
Patients undergoing
fertility treatment,
but still cannot conceive
-
Failure to conceive
even after transfer of
a good embryo
-
Patients with repeated
implantation failure
(RIF)
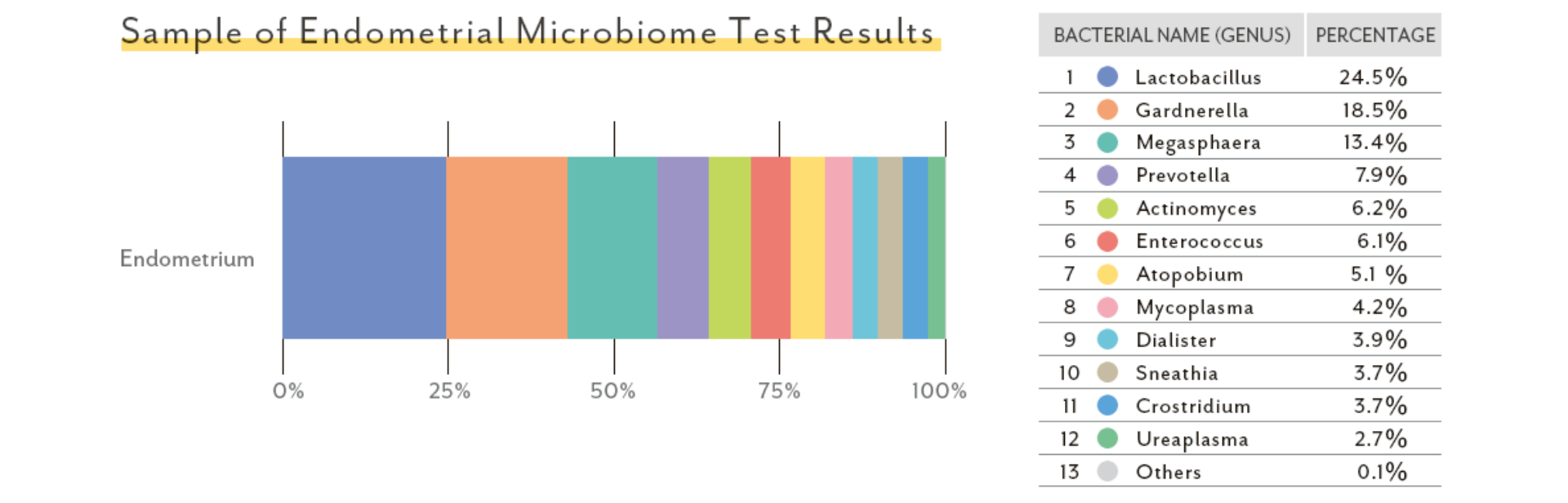
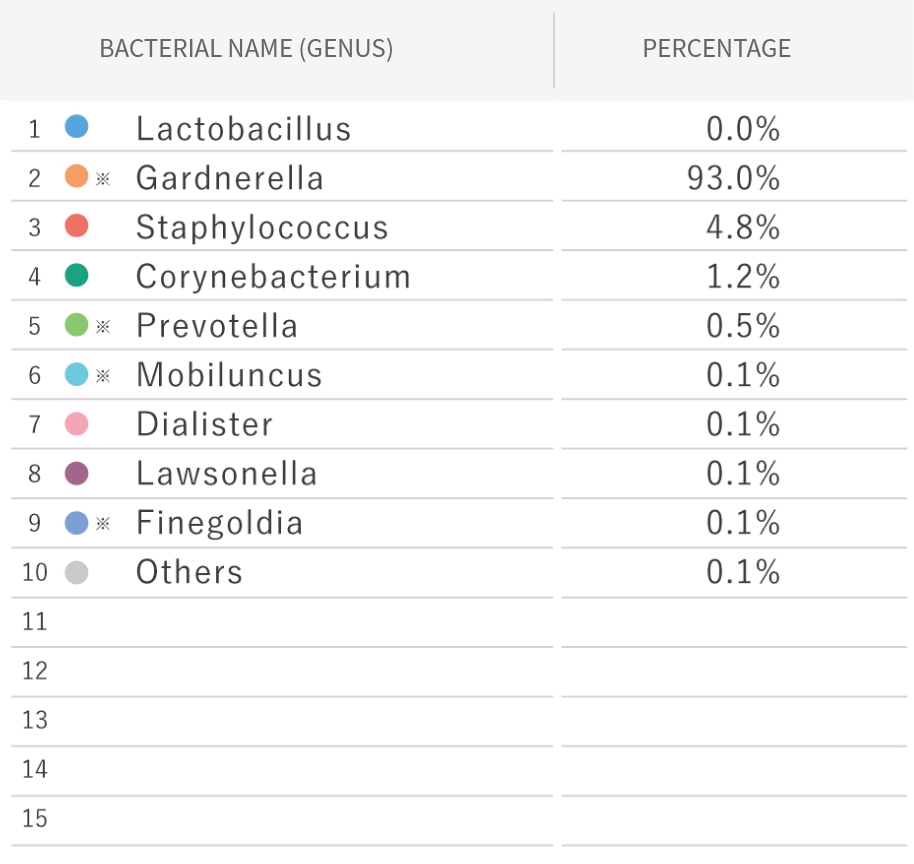
Sample of Endometrial Microbiome Test Results
- 1
- The Lactobacillus spp. percentage
- 2
- The contribution rate of other bacterial species
e.g.Pathogenic bacteria / Opportunistic organismResident microbiota
Sample of Endometrial Microbiome Test
Benefit : Why Endometrial Microbiome Test?
- 1
- Provide endometrial microbiome information and the bacteria in the endometrium
- 2
- Determine the percentage of Lactobacillus in the endometrium
- 3
- Provide patients with information related to treatment
- 4
- Provide contribution rate of other bacterial species including pathogenic bacteria
- 5
- Test failure rate is below 2%
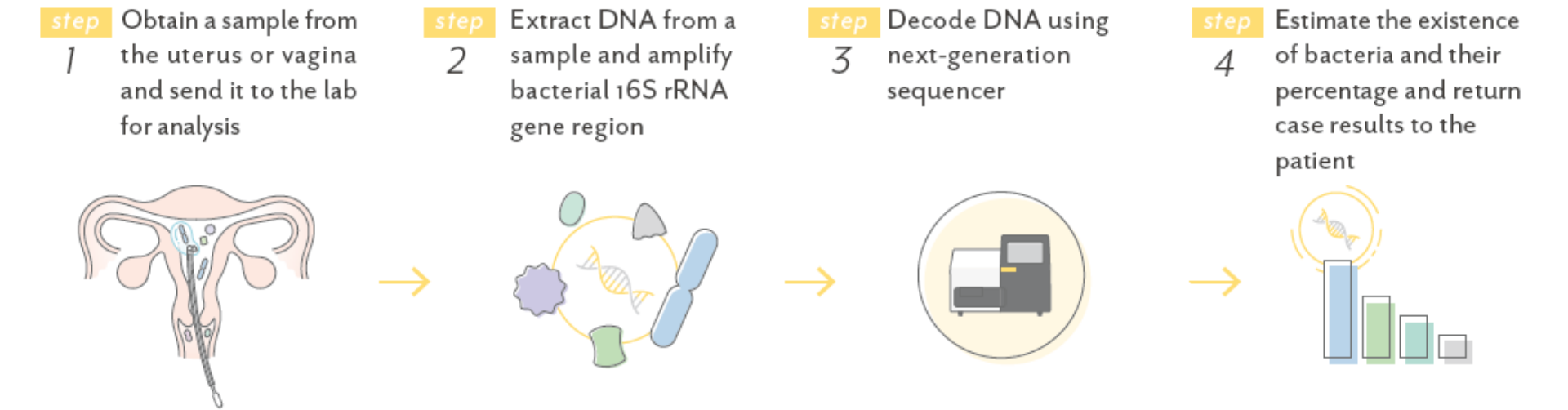
What is the procedure about Endometrial Microbiome Test?
The endometrial microbiome test examines the existence of Lactobacillus spp. in the endometrium or vagina, which is thought to be involved in implantation and pregnancy outcomes.
The test uses a NGS (next-generation sequencer) to analyze the 16Sr RNA genes of all bacteria present in the specimen to identify and estimate the percentage of bacteria present in the specimen. The percentage of other bacterial species including pathogenic bacterial species are also provided.
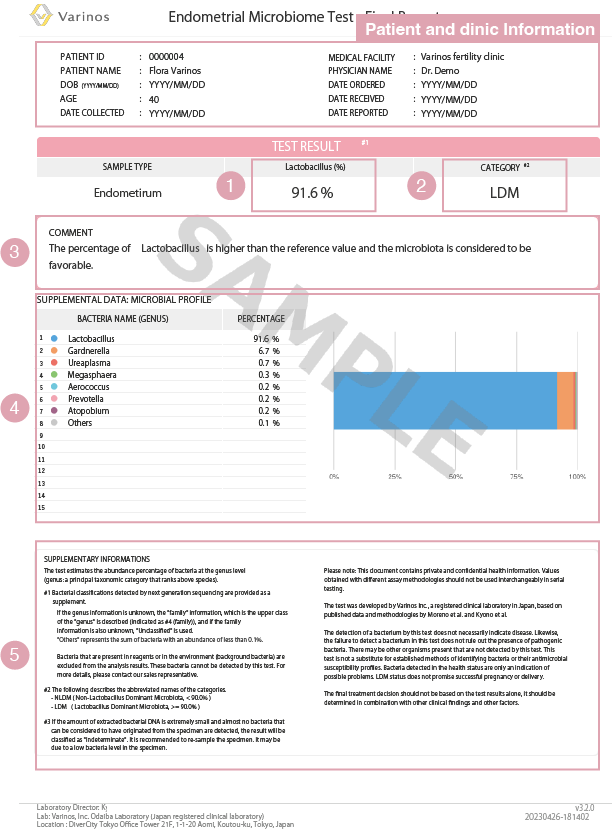
Sample of Endometrial Microbiome Test Results
① Lactobacillus Percentage
The Lactobacillus spp. percentage is provided.
② Category and Evaluation
・The evaluation is based on the Lactobacillus percentage.
LDM:(Lactobacillus Dominant Microbiota): Lactobacillus percentage isgreater than 90%
・NLDM:(Non-Lactobacillus Dominant Microbiota): Lactobacillus Dominant Microbiota is less than 90%.
③ Comments on Test Results
・These comments are for reference only. The final decision on treatment should not be based solely on this result, but should be made in conjunction with o ther clinical or findings.
・No comments are available for specimens not subject to determination.
④ Bacterial Profile and Microbiota Graph (Reference)
・f the specimen is not eligible for determination, this area will be blank
・Please make clinical decision s in conjunction with other clinical findings.
⑤ Notes on the Test Report
Explanation of how to read the test results.
This test estimates the abundance ratio of bacteria at the genus level. The genus is a group of species, and is the smallest unit in the classification of organisms.
Bacteria that are already present in reagents or in the environment (background bacteria) are excluded from the test results. These types of bacteria cannot be detected by this test.
Documentation and Resources
Scientific publications
Y.Shi et al.J Reprod Immunol. 2022 Aug;152:103653. doi: 10.1016/j.jri.2022.103653.
M.Miyagi et al. JBRA Assist Reprod. 2022 Dec 5. doi: 10.5935/1518-0557.20220040.
T.Ichiyama et al. Reprod Med Biol. 2021 May 31;20(3):334-344.
K.Kyono et al. Reprod Med Biol. 2018 Oct 25;18(1):72-82.
Moreno, Inmaculada et al. American Journal of Obstetrics & Gynecology, Volume 215, Issue 6, 684 - 703.