Medical Venture Varinos Raises 600 Million Yen in Series C
Accelerating global expansion of “Endometrial Microbiome Test” and R&D of new products
Varinos Inc., which develops genetic tests using genomic technology (headquartered in Koto-ku, Tokyo; Yoshiyuki Sakuraba, CEO; Varinos), has raised 600 million yen in a Series C round funding.In addition to existing investors Angel Bridge, SMBC Venture Capital, and Miyako Capital, we have added four new underwriters: Fast Track Initiative, Mitsubishi UFJ Capital, MTG Ventures, and Sony Innovation Fund.
This brings the total amount raised to 1.1 billion yen since our establishment.
With the funds raised this time, we plan to invest in the global development of the Endometrial Microbiome Test (*1), which is being introduced in the field of infertility treatment, and in the R&D of new products based on the Endometrial Microbiome Test technology.
Recently, “genomic medicine,” in which treatment is based on individual genome information, has been attracting attention in Japan and abroad, and it is expected that genome information will be utilized more in the medical field in the future.
Varinos aims to become a world-leading company not only capable of analyzing genomes, but also of developing new tests that utilize genome technology, as a company originating in Japan.
※1 – The world’s first test was developed and implemented by Varinos in December 2017 by applying genome technology.
Varinos’ business and future development
Varinos is one of the few Japanese companies that can develop not only advanced genome analysis but also tests using genome technology.We were the first in the world to develop the “Endometrial Microbiome Test,” which detects ultra-low biomass of bacteria in the endometrium, and put it to practical use as a clinical test. Currently, we are developing our business by applying genome technology in the field of infertility treatment.In particular, the “Endometrial Microbiome Test,” our core business, has been introduced at more than 250 medical institutions, mainly fertility clinics in Japan, since it is known that the bacterial environment in the uterus affects the pregnancy rate.
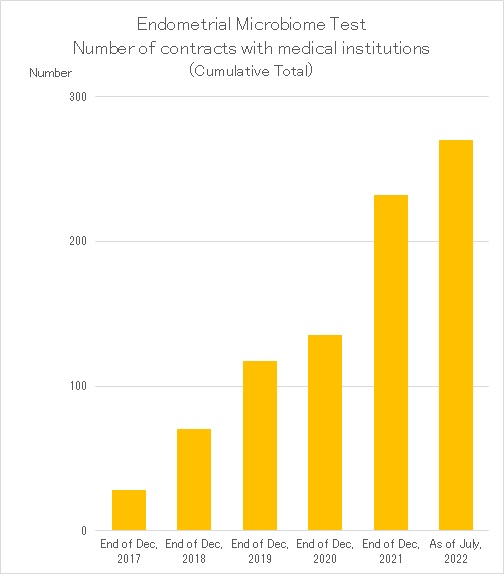
In April 2022, insurance coverage of infertility treatment was expanded in Japan, and while the number of people undergoing infertility treatment is expected to increase, our “Endometrial Microbiome Test” was approved as an advanced medical treatment in July, allowing it to be used in combination with insurance treatment.In Japan, we will continue to provide tests in order to help doctors and patients who are seeking infertility treatment.
・Global Expansion of Endometrial Microbiome Test
Endometrial Microbiome Test requires advanced technologies and is still in practical use by only a few companies overseas, .Therefore, we will continue to provide the Endometrial Microbiome Test in cooperation with medical institutions not only in Japan but also overseas.
・R&D of new products based on endometrial microbiome technology
In addition to its relationship with pregnancy rates, research has begun to suggest that endometrial microbiome is also associated with chronic endometritis, endometriosis, and cervical cancer.We will also focus on disease research where we can apply our i endometrial microbiome technology and utilize genomic information to develop new products.
◆Investors in this round
All three existing investors made additional investments.Four new investors have been added.
[New investors]
・Fast Track Initiative, Inc. (lead investor)
・Mitsubishi UFJ Capital Co., Ltd.
・MTG Ventures
・Sony Innovation Fund
[Existing Investors]
・Angel Bridge
・SMBC Venture Capital Co., Ltd.
・Miyako Capital
◆About the Endometrial Microbiome Test
In 2015, researchers at Rutgers University in the US overturned the common belief that there are no bacteria in the uterus, and in 2016, a study at Stanford University in the US found that disruption of intrauterine flora (bacterial environment in the uterus) reduces the success rate of in vitro fertilization.
We focused on the fact that there is a correlation between the percentage of Lactobacillus spp. in the intrauterine flora and the pregnancy rate, and in December 2017, we originally developed and commercialized the world’s first “Endometrial Microbiome Test” as a clinical test.
This test enables a comprehensive examination of lactobacilli and pathogenic bacteria in the uterus that may cause infertility or implantation failure of fertilized eggs.
